Vision Checks Optical Characteristics of Artificial Corneas
Thousands of low-vision patients could benefit from corneal grafts each year. However, with a limited supply of donor corneas, synthetic alternatives have become medically essential. Oxford MEStar, a bioengineering company, produces artificial corneas to address this need.
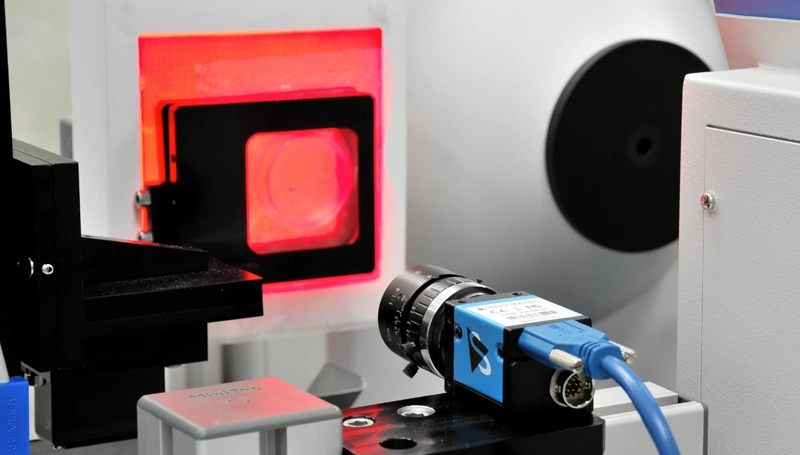
Gary Livingstone, Managing Director of LG Motion, explains how his firm developed a semi-automated inspection system for MEStar. This solution tackles the challenge of quality control for delicate transparent prostheses. The vision-based motion control system incorporates The Imaging Source’s DMK 23UP031 USB 3.0 industrial camera.
Fig. 1: LG Motion’s Vision-Based Inspection System
The system features The Imaging Source’s DMK 23UP031 monochrome USB 3.0 camera designed for precise optical analysis.
Translucency Challenges in Inspection
Artificial corneas undergo strict medical controls, particularly transparency measurements via laser analysis. However, this very characteristic complicates automated inspection processes. Corneas are suspended in a transparent medium within Petri dishes and packaged in sterile blister packs (Fig. 2).
The challenge lies in locating the transparent cornea within its packaging without human intervention.
Fig. 2: Packaging Design
Artificial corneas and their support scaffolds are suspended in a transparent gel and sealed in clear plastic blister packs for sterility.
Technical Solution
The inspection system employs structured light technology combined with machine vision algorithms:
-
Samples are illuminated by patterned red LED lighting (0.25mm grid pattern)
Fig. 3: Illumination Setup
The inspected samples receive a patterned, red LED illumination featuring a 0.25mm grid pattern.
-
Scorpion Vision’s Compact System analyzes the resulting distortions to identify edges
-
Monochrome imaging enables precise edge detection and geometric analysis
Semi-Automation Implementation
The system uses an Arduino processor integrated with Arcus Technology stepper motor controllers for automated decision-making:
- Defective items are diverted through rejection chutes
- Acceptable products proceed through acceptance channels
- The continuous inline production process maintains high throughput while ensuring quality standards.
This technical solution demonstrates how advanced vision systems can address complex manufacturing challenges in medical device production.
Last Updated: 2025-09-05 00:55:28